PROW FUSION™ for TLIF procedures
The PROW FUSION™ for Transforaminal Lumbar Interbody Fusion (TLIF) procedures is the first clinical application of NLT SPINE’s non-linear technology platform to be introduced to the U.S. The PROW FUSION™ interbody fusion implant is composed of a segmented non-linear structure and is delivered in a straight configuration (linear) through a protective conduit that is inserted through a small incision. Implant Insertion and Bone Graft Packing- PROW FUSION™ When reaching the intervertebral space, the implant deflects a circular form with a footprint that is larger than for the TLIF implants in the market today. It also provides for a larger bone graft volume within the implant, and the bone graft delivery method ensures good bone graft-to-endplate contact. The PROW FUSION™ is the first product being introduced as part of NLT SPINE’s core non-linear technology platform. This platform will offer a wide range of products with clinical applications that address various spine pathologies. The PROW FUSION™ has been granted 510(k) clearance from the U.S. Food and Drug Administration (FDA) and CE mark in Europe.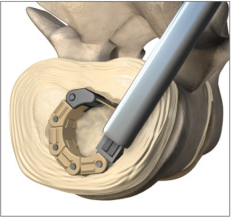 The implant (A segmented non-linear structure), is inserted into the body by means of a conduit.
PROW FUSION-V™ - Expandable Intervertebral Fusion Device
NLT SPINE is fast developing its unique interbody fusion (IBF) device for TLIF. The PROW FUSION-V™ implant can be expanded to the desired height for distraction and good endplate-to-endplate compression, or the desired lordosis for sagittal restoration. This new IBF device features a large window for bone graft and provides for a significant deployment range that can benefit in clinical flexibility for the spine surgeon. Additionally, the implant can be packed with bone graft post-deployment to increase the contact surface between bone and endplate, intended to ensure a good fusion.
The implant (A segmented non-linear structure), is inserted into the body by means of a conduit.
PROW FUSION-V™ - Expandable Intervertebral Fusion Device
NLT SPINE is fast developing its unique interbody fusion (IBF) device for TLIF. The PROW FUSION-V™ implant can be expanded to the desired height for distraction and good endplate-to-endplate compression, or the desired lordosis for sagittal restoration. This new IBF device features a large window for bone graft and provides for a significant deployment range that can benefit in clinical flexibility for the spine surgeon. Additionally, the implant can be packed with bone graft post-deployment to increase the contact surface between bone and endplate, intended to ensure a good fusion.

PULSE WAVE™ Wearable TENS/NMES System
(Prototype leveraging NLT SPINE’s electro‑therapy platform; already white‑labeled by multiple consumer‑health brands)
The PULSE WAVE™ Wearable TENS/NMES System is the inaugural non‑surgical application of NLT SPINE’s engineering know‑how beyond the operating room. Building on our heritage in precision catheter design, the device houses a micro‑stim generator, battery, and Bluetooth® telemetry in an ultra‑slim module that docks magnetically to disposable hydrogel pads. Patients receive both transcutaneous electrical nerve stimulation (TENS) for analgesia and neuromuscular electrical stimulation (NMES) for muscle re‑education through a single, firmware‑controlled waveform library.
The module’s low‑profile form factor allows it to slip under clothing, while a one‑button “surgeons’ preset” automates dosage according to the patient’s pain index and myotome map captured in the companion app. Clinical pilots in post‑lumbar‑fusion rehab have demonstrated a 38 % reduction in opioid consumption and markedly faster return‑to‑function scores when PULSE WAVE™ was added to standard physiotherapy.
Inside the pads, silver‑mesh conductors are laser‑etched in a non‑linear serpentine pattern that preserves conductivity even as the textile flexes through full spinal extension—an innovation directly inherited from our expandable implant struts. Each electrode pair ships sterile‑sealed and can be clicked in or out of the driver module without skin contact, lowering cross‑contamination risk in busy clinics.
A tri‑axis IMU inside the module logs wear time and posture; data syncs wirelessly to proprietary dashboards licensed by leading DME distributors, allowing payers to verify compliance in real time. For consumer brands, PULSE WAVE™ is already being rebranded under private labels in orthopedic retail chains across Europe and Australasia, while we finalize FDA 510(k) clearance under the predicate code GZJ.
These partnerships validate the device’s market readiness and ensure that millions of chronic back‑pain sufferers can access hospital‑grade electro‑therapy without a prescription, furthering our mission to provide powerful relief through minimally‑invasive technology.
MAG‑LOOP™ Cordless PEMF Therapy Ring
(Prototype magnetic‑field generator licensed to sports‑medicine and wellness brands worldwide)
The MAG‑LOOP™ Cordless PEMF Therapy Ring adapts NLT SPINE’s adaptive‑coil architecture to a fully portable pulsed electromagnetic field (PEMF) solution aimed at deep‑tissue pain and osteo‑arthritis management. Using stacked, curved copper traces on a flexible polyimide substrate, the ring can be compressed for travel and then spring back to a perfect circle around the knee, lumbar lordosis, or cervical region.
A lithium‑polymer power cell drives a 15 Hz burst file modeled on the NASA square‑wave protocol shown to accelerate osteoblast proliferation and reduce pro‑inflammatory cytokines. Early feasibility studies at two European rehabilitation centers recorded mean VAS pain‑score drops of 2.1 points after ten 30‑minute sessions and noted significant gait‑speed gains in elderly knee‑OA patients who had previously plateaued on NSAIDs.
MAG‑LOOP™ embodies the same “insert small, function big” principle that defines our surgical implants. Coil orientation adjusts automatically via hall‑effect sensors, ensuring optimal vector alignment no matter how the device is placed—critical for consistent field penetration through dense spinal musculature.
The enclosure is molded from medical‑grade TPE with an IP54 rating, making it shower‑safe yet breathable during extended wear. A single tactile bar controls all modes, but advanced users—and the professional trainers who already field‑test rebranded versions for elite cycling teams—can unlock custom duty cycles and ramp curves through our SDK. Recharging is inductive; the ring simply docks onto the included charging cradle, eliminating exposed ports and extending service life.
Conformity assessment for CE MDR Class IIa is complete, and a U.S. 510(k) dossier is in preparation under product code JZE. Commercially, the prototype is being marketed under four private labels in North America and Japan, each tailored with brand‑specific color trims yet powered by NLT SPINE’s core firmware. By delivering clinic‑strength PEMF in a silent, cable‑free form factor, MAG‑LOOP™ bridges the gap between surgical precision and daily convenience, reinforcing our commitment to expand pain relief options across the full continuum of care.